The Influence of Omega-3 Fatty Acids on Semen Quality Markers a Systematic Prisma Review

Creatine as a Promising Component of Paternal Preconception Diet
one
Section of Diet and Public Health, Academy of Agder, P.O. Box 422, 4604 Kristiansand, Kingdom of norway
two
FSPE Applied Bioenergetics Lab, University of Novi Sad, 21000 Novi Sad, Serbia
3
Section of Health and Nursing Sciences, University of Agder, P.O. Box 422, 4604 Kristiansand, Norway
4
Department of Child and Adolescence Mental Wellness, Sørlandet Infirmary, 4604 Kristiansand, Norway
*
Author to whom correspondence should be addressed.
Bookish Editors: Annalisa Terranegra and Cinzia Myriam Calabrese
Received: 11 January 2022 / Revised: 19 Jan 2022 / Accepted: 20 Jan 2022 / Published: 28 January 2022
Abstruse
Male fertility has been declining globally over the past several decades, advancing from a personal effect to a public health trouble. Across whatever doubt, a reduction in fertility (oft characterized by low sperm count or motion) can severely threaten reproductive health and lifecourse framework in a long-term mode. Aside from uncovering the currently unknown etiology of modern-twenty-four hours male infertility, the scientific and medical community faces a double burden: finding an efficient biomarker of impaired fertility and exploring any intervention that tin can act to enhance fertility. A plethora of nutritional compounds have been recognized as possible modulators of semen quality, and specific dietary patterns and nutrients announced to be accompanied by a lower risk of male person infertility. Creatine, a conditionally essential nutrient, has caught attention as a male fertility-promoting candidate due to its role in sperm energy metabolism. This mini-review describes the creatine-related bioenergetics of spermatozoa, explores a connection between creatine levels and sperm quality in men, and critically examines available evidence for interventional studies with creatine to affect sperm viability.
1. Background
According to the Global Brunt of Illness study, global fertility rates have been dropping steadily whereas life expectancy has been increasing over the past twenty years [1]. Although the factors behind this worldwide refuse in fertility rate remain largely unclear [2], a driblet in semen quality represents a significant public health issue in terms of reproductive and lifecycle health [three]. Likewise other factors, poor nutrition has been recognized as a possible disruptor of semen quality, and the Western-style nutrition appears to be accompanied past a college risk of male infertility [iv,five]. Healthy dietary patterns, on the other manus, correlate well with better sperm milieu, and a smaller gamble of abnormalities in parameters such as sperm count, sperm concentration, and motility [vi]. Whole-nutrition interventions, such equally the Mediterranean diet [seven] or plant-based diet [eight], as well equally individual nutrients, including zinc [9], selenium [10], and omega-3 fat acids [11], are put forward as nutritional models that could support male fertility. Recognizing other dietary interventions able to raise sperm quality and support paternal preconception capacity remains of the utmost interest for both the research community and the full general public. Moreover, as male infertility is a complex biological and social miracle, identification of valid biomarkers for infertility diagnosis has been requested [12]. Creatine, a conditionally essential nutrient and a popular dietary supplement [thirteen], drives attention every bit some other male fertility-promoting candidate due to its function in sperm energy metabolism. This mini-review describes creatine-related bioenergetics of spermatozoa, explores a connection between creatine levels and sperm quality in men, and critically examines bachelor evidence for interventional studies with creatine to impact sperm viability.
ii. Semen: An Free energy-Demanding Fluid
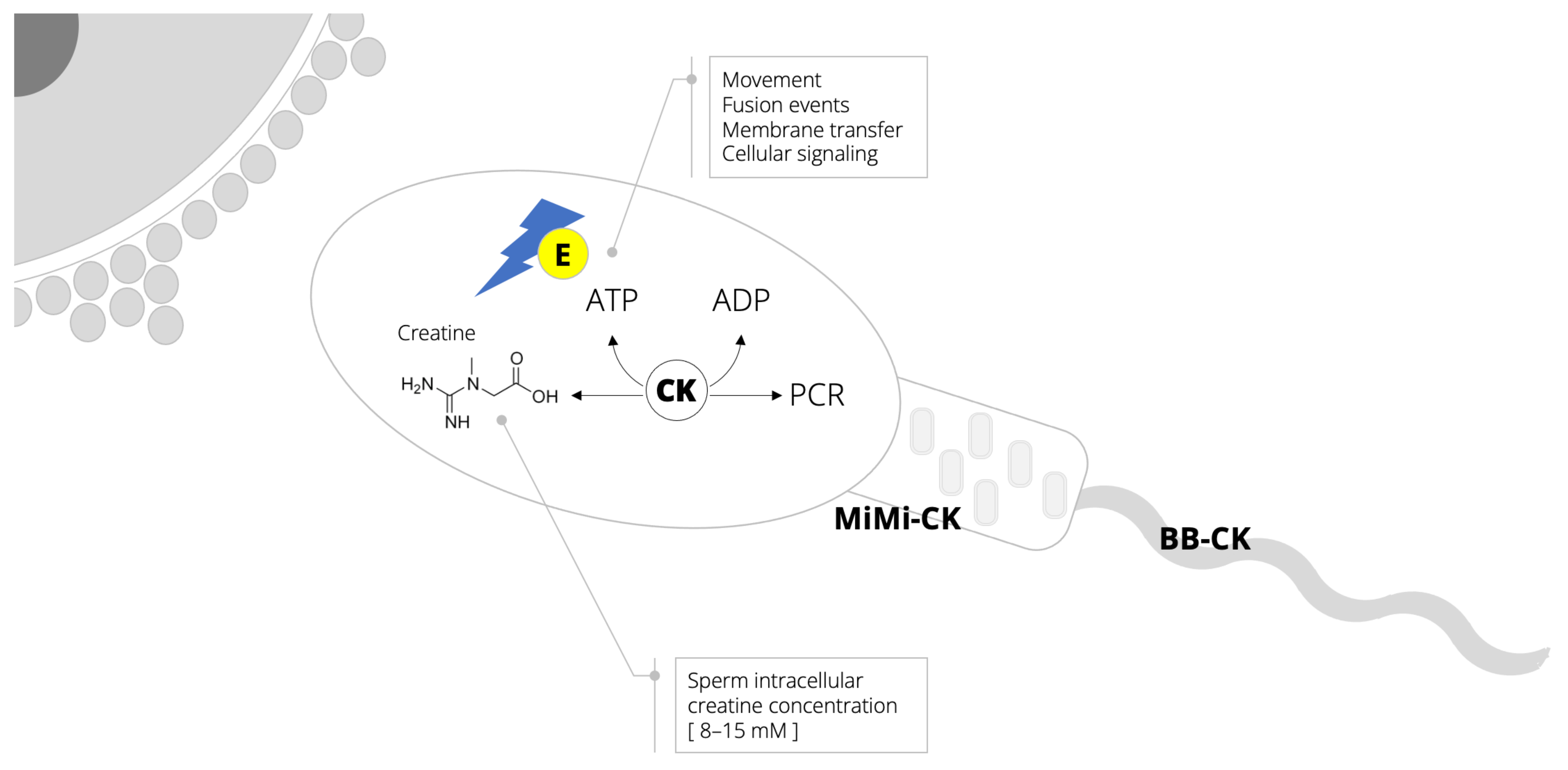
As a male reproducing fluid that predominantly contains spermatozoa (along with various organic and inorganic compounds), semen exhibits infrequent resilience to withstand environmental stress and promote the survival of cells prior to and during conception. This is more often than not due to the fact that spermatozoa can effectively sustain high and fluctuating energy requirements [xiv]. A continuous supply of high-free energy phosphates in sperm requires a significant contribution of the creatine–phosphocreatine shuttle (Figure 1), a critical metabolic pathway in cellular bioenergetics [15]. Total creatine content of spermatozoa (8–15 mM) and seminal plasma (~4 mM) [sixteen,17] are comparable to levels found in other energy-enervating cells, such as skeletal and cardiac myocytes, and photoreceptor cells of the retina [18]. Amidst unlike functions, creatine in spermatozoa is involved in the phosphocreatine shuttle, thereby shuttling energy (adenosine triphosphate, ATP) from the mitochondria to the contractile machinery to fuel motion just likewise fertilization, cellular send, and other metabolic reactions. Creatine kinase is also indispensable for sperm function considering information technology catalyzes the regeneration of free energy from the shuttle [19]. Sperm high-free energy production is compartmentalized, with two distinct creatine kinase isoenzymes found in the sperm tail and midpiece region rich in mitochondria [17]. Interestingly, the inactivation of creatine kinase can impair the pattern of sperm motility [xx]. Having this in mind, the evaluation of creatine–phosphocreatine shuttle biomarkers is often used every bit a tool to monitor sperm health [16,21] (Box i).
Box 1. Sperm quality vs. fertility.
Previous studies accept used both sperm quality and fertility interchangeably, and therefore the terms are used in the same way in this mini-review. All the same, the reader should exist aware that the apply of sperm quality as a mark for male fertility is highly debated. Sperm quality mainly concerns the number of spermatozoa and their viability, motility, and morphology. Although these are all factors that lower the gamble of conception, they are rarely the but cause of infertility. Other factors, such as the human's age or his partner's age, may be contributing factors to the success of formulation. Thus, poor sperm quality does not necessarily predict infertility [22].
3. Biomarkers of Creatine Metabolism and Sperm Quality
A possible link between semen creatine metabolism and sperm quality in humans has been debated for near threescore years. In a preliminary communication published in The Lancet back in 1963, Lehmann and Griffiths [23] suggested that extremely high concentrations of creatine kinase found in seminal fluid (385–14,000 IU) might be used in tracking azoospermia. This seminal article was followed by a handful of reports describing creatine kinase levels in fertility studies [24,25,26], with nearly all suggesting a relative value of the enzyme activity as an indicator of spermatogenesis. Arguably the outset report nigh the seminal concentration of creatine and sperm viability was reported by Srivastava and co-workers [27]. The authors demonstrated that creatine levels tended to be higher in normal males than in infertile counterparts, suggesting the remarkable importance of creatine for spermatozoa quality. Huszar et al. [28] confirmed qualitative metabolic differences amidst the sperm of oligospermic and normospermic men, with a highly significant inverse correlation betwixt sperm creatine kinase activities and sperm concentrations. The inverse relationship between creatine kinase levels and sperm concentration and morphology was found in sub-fertile men, implying that elevated creatine kinase levels may reflect biochemically immature spermatozoa [29]. Some other written report found that the mean creatine kinase levels in the severely oligospermic group were eighteen-fold higher than that in the moderate and balmy groups, with creatine kinase higher in all three infertile groups compared with the donor group [30]. Interestingly, the concentration of two isoforms of creatine kinase (CK-B and CK-M) in normozoospermic and 2 groups of oligozoospermic patients were significantly unlike, with CK-M levels correlated negatively with sperm concentration and sperm motility, but correlated positively with the pathologic sperm form [31]. A recent trial reported that low semen creatine levels are associated with reduced sperm move, while high creatine kinase action is associated with poor sperm quality [16]. Interestingly, diverse lifestyle factors can impair sperm bioenergetics and creatine kinase activeness, every bit well as sperm motion, including smoking [32] and environmental exposure to pesticides [33]. The above findings suggest a relationship between compromised creatine–phosphocreatine metabolism and depression sperm count/activity; recovering normal creatine turnover in spermatozoa thus might help males with poor quality sperm.
four. Exogenous Creatine and Sperm Viability
Utilizing exogenous creatine to better sperm quality has been investigated in a handful of in vitro and creature studies and so far. The add-on of creatine phosphate to the insemination media enhances the fertilizing capacity of sperm (both motion and velocity) during in vitro fertilization [34]. Creatine also enhances sperm capacitation by increasing adenosine triphosphate levels when added to in vitro fertilization medium [35]. Indeed, successful fertilization was achieved with as few as five sperm in the creatine group, and the number of fertilized oocytes was significantly college than in the control grouping without creatine. Creatine induced and sustained zig-zag sperm motility and improved the fertilization ability of boar sperm under hypoxic weather when added to in vitro fertilization medium [36]. A dietary administration of creatine forerunner, guanidinoacetic acid, was associated with the improvement in semen concentration, total sperm number, and sperm forrad move (also sperm penetration and fertility rate) in broiler breeder roosters [37]. An interesting cross-sectional study in 778 young, healthy men taking poly peptide supplements (of those, 44% men reported using creatine) found that semen concentration and total sperm count tended to exist college in current users than in never users (42 vs. 36 million/mL, and 108 vs. 90 1000000, respectively) [38]. Although preliminary, the in a higher place studies provided the first testify most the potential effects of exogenous creatine in tackling sperm quality; this strongly justifies further interventional and mechanistic studies with dietary creatine in a real-life context of male (in) fertility. Specifically, information technology remains unknown how dietary creatine is delivered to spermatozoa, and whether infertility may compromise creatine uptake. Preclinical studies demonstrate an expression of testis-specific creatine transporter (CT2) [39,twoscore], with its office in creatine uptake (sequestration of creatine from the plasma and/or creatine transport inside the reproductive tract) remains to be clarified.
5. Paternal Preconception Diet with Creatine: The Future Steps
Building male person fertility through the diet might be a simple, convenient, and straightforward strategy to tackle this fundamental element of reproductive health. Working towards this goal requires many studies employing various dietary routines and nutrients, and creatine could be the next promising agent in the pipeline. In terms of lifecycle nutrition, dietary creatine has been confirmed as a particularly of import chemical compound in female reproduction, pregnancy, and newborn health (for a detailed review, meet Ref. [41]), and for the normal growth of children and adolescents [42]. Dietary intake of creatine in very young children (0–24 months) is roughly three times larger than that of the developed population [43], implying its critical role in optimal brain development for this sensitive population. A summary of experimental studies suggests a protective role of maternal peri-conception diet complemented with creatine to improve fetal and neonatal morbidity and reduce bloodshed in high-risk human pregnancy [44]. Whether a dietary intake of creatine of father at the conception, and even before, influences fertility biomarkers (along with the wellness of their future children) currently remains unknown. A first pace in addressing this question might require re-evaluating fertility cohort data by exploring possible associations between the intake of creatine-containing foods (eastward.g., fish, meat, milk) and fertility biomarkers in men, followed by well-designed long-term randomized controlled trials with dietary creatine.
6. Conclusions
As energy-enervating cells, spermatozoa can endure from deficient creatine metabolism, with poor sperm count and motility are often associated with low creatine levels. A provision of supplemental creatine appears to positively affect sperm quality in pilot trials, suggesting a potential for using creatine to attenuate sub-fertility. This is accompanied by favorable safety profile of creatine supplementation reported in the scientific and medical literature then far, with short and long-term supplementation (up to thirty g/mean solar day for 5 years) is safe and well-tolerated in salubrious individuals and in a number of patient populations ranging from infants to the elderly [45]. All the same, to get recognized as a functional component of a paternal preconception diet, dietary creatine has a long journeying alee that should commencement with exploring its intervention in men with depression sperm concentration.
Writer Contributions
Due south.M.O. designed and wrote the manuscript and has primary responsibility for the last content; and S.Chiliad.O., T.H.S. and D.Due east. read, revised and approved the last version of the manuscript. All authors accept read and agreed to the published version of the manuscript.
Funding
This work was non funded by whatever agency in the public, commercial, or not-for-turn a profit sectors.
Institutional Review Board Argument
Not applicable.
Informed Consent Statement
Not applicative.
Data Availability Statement
Non applicable.
Conflicts of Interest
S.G.O. serves equally a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). S.M.O. owns patent "Sports Supplements Based on Liquid Creatine" at European Patent Office (WO2019150323 A1), and active patent application "Synergistic Creatine" at UK Intellectual Holding Office (GB2012773.4). Due south.G.O. has served every bit a speaker at Abbott Nutrition, a consultant of Centrolineal Beverages Adriatic and IMLEK, and has received enquiry funding related to creatine and/or guanidinoacetic acid from the Serbian Ministry of Education, Scientific discipline, and Technological Evolution, Provincial Secretariat for Higher Didactics and Scientific Inquiry, AlzChem GmbH, KW Pfannenschmidt GmbH, Hueston Hennigan LLP, and ThermoLife International LLC. S.M.O. does not own stocks and shares in any arrangement. T.H.S. is a member of the Norwegian Scientific Committee for Food Safety and has contributed to a gamble assessment of creatine as nutrient supplement (Opinion of the Panel of Diet, Dietetic Products, Novel Nutrient and Allergy Report 2016:56). D.East. declares no conflict of interest.
References
- GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, good for you life expectancy (Unhurt), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1160–1203. [Google Scholar] [CrossRef]
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male person fertility. Curr. Opin. Urol. 2020, xxx, 296–301. [Google Scholar] [CrossRef]
- Virtanen, H.E.; Jørgensen, N.; Toppari, J. Semen quality in the 21st century. Nat. Rev. Urol. 2017, 14, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Chavarro, J.E.; Tanrikut, C. Diet and men'southward fertility: Does diet affect sperm quality? Fertil. Steril. 2018, 110, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Salas-Huetos, A.; James, Eastward.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, 50.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and nutritional factors in male (in)fertility-underestimated factors. J. Clin. Med. 2020, ix, 1400. [Google Scholar] [CrossRef]
- Karayiannis, D.; Kontogianni, M.D.; Mendorou, C.; Douka, L.; Mastrominas, Grand.; Yiannakouris, N. Association betwixt adherence to the Mediterranean diet and semen quality parameters in male person partners of couples attempting fertility. Hum. Reprod. 2017, 32, 215–222. [Google Scholar] [CrossRef]
- Braga, D.P.; Halpern, G.; Figueira Rde, C.; Setti, A.Southward.; Iaconelli, A., Jr.; Borges, East., Jr. Nutrient intake and social habits in male patients and its human relationship to intracytoplasmic sperm injection outcomes. Fertil. Steril. 2012, 97, 53–59. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc is an essential chemical element for male fertility: A review of Zn roles in men's health, germination, sperm quality, and fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Scott, R.; MacPherson, A.; Yates, R.W.; Hussain, B.; Dixon, J. The upshot of oral selenium supplementation on human sperm motion. Br. J. Urol. 1998, 82, 76–lxxx. [Google Scholar] [CrossRef]
- Falsig, A.L.; Gleerup, C.Due south.; Knudsen, U.B. The influence of omega-3 fatty acids on semen quality markers: A systematic PRISMA review. Andrology 2019, vii, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, Due south.A.; Cisneros, P.; Steinkampf, M.P.; Colina, J.A.; et al. Sperm morphology, motion, and concentration in fertile and infertile men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Forbes, S.C. Perspective: Creatine, a conditionally essential nutrient: Edifice the case. Adv. Nutr. 2021, 18, nmab111. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E. Sperm bioenergetics in a nutshell. Biol. Reprod. 2012, 87, 72. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Tokarska-Schlattner, Chiliad.; Schlattner, U. The creatine kinase arrangement and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef]
- Nasrallah, F.; Hammami, M.B.; Omar, S.; Aribia, H.B.; Sanhaji, H.; Feki, Thou. Semen creatine and creatine kinase activity as an indicator of sperm quality. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Wallimann, T.; Moser, H.; Zurbriggen, B.; Wegmann, Thousand.; Eppenberger, H.K. Creatine kinase isoenzymes in spermatozoa. J. Musculus Res. Cell Motil. 1986, 7, 25–34. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, lxxx, 1107–1213. [Google Scholar] [CrossRef]
- Banihani, S.A.; Abu-Alhayjaa, R.F. The activity of seminal creatine kinase is increased in the presence of pentoxifylline. Andrologia 2016, 48, 603–604. [Google Scholar] [CrossRef]
- Tombes, R.M.; Shapiro, B.M. Metabolite channeling: A phosphorylcreatine shuttle to mediate high energy phosphate send between sperm mitochondrion and tail. Cell 1985, 41, 325–334. [Google Scholar] [CrossRef]
- Grow, D.; Oehninger, Due south. Strict criteria for the evaluation of human sperm morphology and its touch on assisted reproduction. Andrologia 1995, 27, 325–333. [Google Scholar] [CrossRef]
- Patel, A.S.; Leong, J.Y.; Ramasamy, R. Prediction of male person infertility by the Earth Health System laboratory manual for cess of semen analysis: A systematic review. Arab. J. Urol. 2017, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.; Griffiths, P.D. Creatine-phosphokinase activeness in semen. Lancet 1963, 2, 498. [Google Scholar] [CrossRef]
- Gonzalez Buitrago, J.M.; Miralles, J.K.; Muńoz, Grand.H.; Meza, S.; Alonso, M.T.; Garcia Diez, 50.C. Seminal plasma creatine kinase activity in fertility studies. Arch. Androl. 1980, 5, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Asseo, P.P.; Panidis, D.Yard.; Papadimas, J.S.; Ikkos, D.K. Creatine kinase in seminal plasma of infertile men: Activity and isoenzymes. Int. J. Androl. 1981, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Buitrago, J.M.; Garcia Diez, L.C. Enzyme levels in semen of men with different types of azoospermia. Andrologia 1982, fourteen, 77–80. [Google Scholar] [CrossRef]
- Srivastava, A.; Chopra, S.K.; Dasgupta, P.R. Biochemical analysis of human seminal plasma. 2. Protein, non-protein nitrogen, urea, uric acrid and creatine. Andrologia 1984, 16, 265–268. [Google Scholar] [CrossRef]
- Huszar, G.; Corrales, Yard.; Vigue, L. Correlation between sperm creatine phosphokinase activity and sperm concentrations in normospermic and oligospermic men. Gamete Res. 1988, 19, 67–75. [Google Scholar] [CrossRef]
- Sidhu, R.S.; Sharma, R.K.; Agarwal, A. Relationship betwixt creatine kinase action and semen characteristics in subfertile men. Int. J. Fertil. Womens Med. 1998, 43, 192–197. [Google Scholar]
- Hallak, J.; Sharma, R.K.; Pasqualotto, F.F.; Ranganathan, P.; Thomas, A.J., Jr.; Agarwal, A. Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology 2001, 58, 446–451. [Google Scholar] [CrossRef]
- Durutovic, O.; Lalic, N.; Milenkovic-Petronic, D.; Bojanic, N.; Djordjevic, D.; Milojevic, B.; Ladjevic, Northward.; Mimic, A.; Tulic, L.; Dzamic, Z.; et al. The correlation of biochemical and morphologic parameters in the assessment of sperm maturity. Urology 2013, 82, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.A.; Rostami, One thousand. The effect of cigarette smoking on man sperm creatine kinase activeness: Equally an ATP buffering system in sperm. Int. J. Fertil. Steril. 2013, half-dozen, 258–265. [Google Scholar] [PubMed]
- Celik-Ozenci, C.; Tasatargil, A.; Tekcan, M.; Sati, 50.; Gungor, E.; Isbir, K.; Usta, Chiliad.F.; Akar, M.E.; Erler, F. Effect of abamectin exposure on semen parameters indicative of reduced sperm maturity: A study on farmworkers in Antalya (Turkey). Andrologia 2012, 44, 388–395. [Google Scholar] [CrossRef]
- Fakih, H.; MacLusky, N.; DeCherney, A.; Wallimann, T.; Huszar, G. Enhancement of human sperm motility and velocity in vitro: Effects of calcium and creatine phosphate. Fertil. Steril. 1986, 46, 938–944. [Google Scholar] [CrossRef]
- Umehara, T.; Kawai, T.; Goto, M.; Richards, J.South.; Shimada, M. Creatine enhances the duration of sperm capacitation: A novel gene for improving in vitro fertilization with small-scale numbers of sperm. Hum. Reprod. 2018, 33, 1117–1129. [Google Scholar] [CrossRef]
- Umehara, T.; Tsujita, Northward.; Goto, Chiliad.; Tonai, S.; Nakanishi, T.; Yamashita, Y.; Shimada, M. Methyl-beta cyclodextrin and creatine work synergistically under hypoxic conditions to improve the fertilization ability of boar ejaculated sperm. Anim. Sci. J. 2020, 91, e13493. [Google Scholar] [CrossRef] [PubMed]
- Tapeh, R.S.; Zhandi, M.; Zaghari, G.; Akhlaghi, A. Effects of guanidinoacetic acid diet supplementation on semen quality and fertility of broiler breeder roosters. Theriogenology 2017, 89, 178–182. [Google Scholar] [CrossRef]
- Tøttenborg, S.S.; Glazer, C.H.; Hærvig, K.K.; Høyer, B.B.; Toft, G.; Hougaard, 1000.S.; Flachs, E.M.; Deen, L.; Bonde, J.P.East.; Ramlau-Hansen, C.H. Semen quality amidst young salubrious men taking poly peptide supplements. Fertil. Steril. 2020, 114, 89–96. [Google Scholar] [CrossRef]
- Moore, N.P. The distribution, metabolism and function of creatine in the male person mammalian reproductive tract: A review. Int. J. Androl. 2000, 23, iv–12. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H.; Villard, A.M.; Speer, O.; Wallimann, T.; Bachmann, C. Creatine synthesis and ship during rat embryogenesis: Spatiotemporal expression of AGAT, GAMT and CT1. BMC Dev. Biol. 2005, 5, 9. [Google Scholar] [CrossRef]
- Muccini, A.Grand.; Tran, North.T.; de Guingand, D.L.; Philip, K.; Della Gatta, P.A.; Galinsky, R.; Sherman, 50.Due south.; Kelleher, M.A.; Palmer, Grand.R.; Drupe, Thou.J.; et al. Creatine metabolism in female person reproduction, pregnancy and newborn health. Nutrients 2021, thirteen, 490. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Stajer, 5.; Ostojic, S.Grand. Relationship between dietary creatine and growth indicators in children and adolescents aged 2–nineteen years: A cross-exclusive study. Nutrients 2021, thirteen, 1027. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Todorovic, North.; Stajer, V.; Ostojic, S.Chiliad. Dietary intake of creatine in children aged 0–24 months. Ann. Nutr. Metab. 2021, 77, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, H.; Ellery, S.; Ireland, Z.; LaRosa, D.; Snow, R.; Walker, D.Westward. Creatine supplementation during pregnancy: Summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in loftier-risk human pregnancy. BMC Pregnancy Childbirth 2014, 14, 150. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.South.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.K.; Kleiner, Southward.M.; Almada, A.L.; Lopez, H.50. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in do, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef]
Figure 1. Creatine–phosphocreatine (PCR) shuttle and high-phospate energy (E) production and utilization in spermatozoa. Abbreviations: ATP, adenosine triphosphate; ADP, adenosine diphosphate; CK, creatine kinase; MiMi-CK, mitochondrial CK isoform confined to the midpiece region rich in mitochondria; BB-CK, tail-specific CK isoform localized inside the sperm tail only not in the head portion.
Figure ane. Creatine–phosphocreatine (PCR) shuttle and high-phospate free energy (Due east) production and utilization in spermatozoa. Abbreviations: ATP, adenosine triphosphate; ADP, adenosine diphosphate; CK, creatine kinase; MiMi-CK, mitochondrial CK isoform confined to the midpiece region rich in mitochondria; BB-CK, tail-specific CK isoform localized within the sperm tail but not in the head portion.
| Publisher's Annotation: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 past the authors. Licensee MDPI, Basel, Switzerland. This article is an open up access article distributed under the terms and conditions of the Artistic Commons Attribution (CC Past) license (https://creativecommons.org/licenses/by/4.0/).
Source: https://www.mdpi.com/2072-6643/14/3/586/htm
0 Response to "The Influence of Omega-3 Fatty Acids on Semen Quality Markers a Systematic Prisma Review"
Post a Comment