what happens to the radius as an atom changes to an anion ion
Periodic Trends in Ionic Radii
- Page ID
- 619
An understanding of periodic trends is necessary when analyzing and predicting molecular properties and interactions. Common periodic trends include those in ionization free energy, atomic radius, and electron affinity. One such trend is closely linked to atomic radii -- ionic radii. Neutral atoms tend to increase in size downward a group and subtract beyond a period. When a neutral atom gains or loses an electron, creating an anion or cation, the atom's radius increases or decreases, respectively. This module explains how this occurs and how this tendency differs from that of atomic radii.
Shielding and Penetration
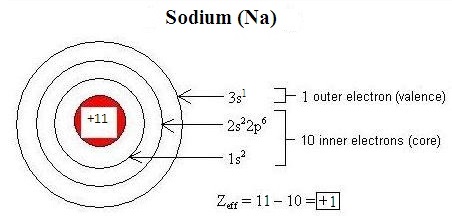
Electromagnetic interactions between electrons in an cantlet modify the effective nuclear accuse (\(Z_{eff}\)) on each electron. Penetration refers to the presence of an electron inside the shell of an inner electron, and shielding is the procedure by which an inner electron masks an outer electron from the full bonny forcefulness of the nucleus, decreasing \(Z_{eff}\). Differences in orbital characteristics dictate differences in shielding and penetration. Within the aforementioned energy level (indicated by the principle breakthrough number, due north), due to their relative proximity to the nucleus, s-orbital electrons both penetrate and shield more effectively than p-orbital electrons, and p electrons penetrate and shield more effectively than d-orbital electrons. Shielding and penetration along with the effective nuclear charge decide the size of an ion. An overly-simplistic only useful conceptualization of constructive nuclear charge is given by the following equation:
\[Z_{eff} = Z - South\]
where
- \(Z\) is the number of protons in the nucleus of an atom or ion (the atomic number), and
- \(S\) is the number of core electrons.
Figure \(\PageIndex{one}\) illustrates how this equation can be used to estimate the constructive nuclear charge of sodium:
Cations and Anions
Neutral atoms that have lost an electron showroom a positive accuse and are chosen cations. These cations are smaller than their respective atoms; this is because when an electron is lost, electron-electron repulsion (and therefore, shielding) decreases and the protons are better able to pull the remaining electrons towards the nucleus (in other words, \(Z_{eff}\)increases). A second lost electron further reduces the radius of the ion. For instance, the ionic radius of Fe2 + is 76 pm, while that of Fe3 + is 65 pm. If creation of an ion involves completely emptying an outer shell, then the decrease in radius is particularly great.
Neutral atoms that take gained an electron are chosen anions, and they are much larger than their respective atoms. Every bit an additional electron occupies an outer orbital, in that location is increased electron-electron repulsion (and hence, increased shielding) which pushes the electrons farther apart. Considering the electrons now outnumber the protons in the ion, the protons tin can not pull the extra electrons as tightly toward the nucleus; this results in decreased \(Z_{eff}\) .
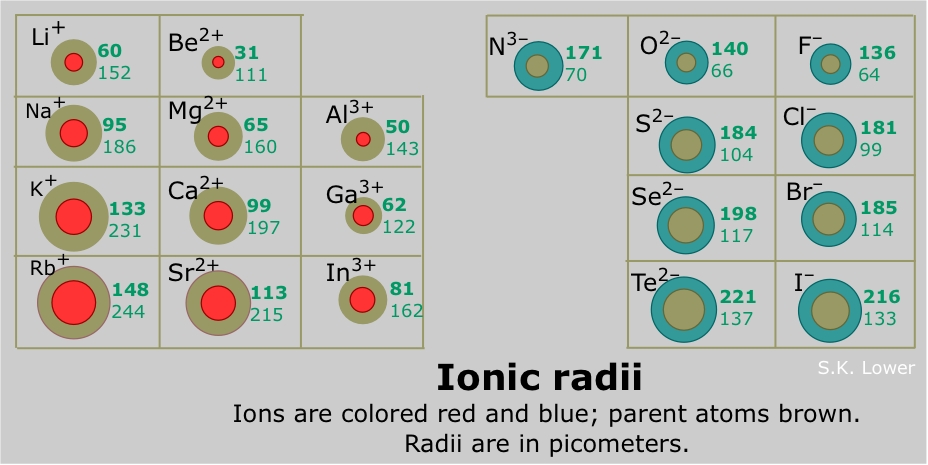
Figure ii shows an isoelectric series of atoms and ions (each has the same number of electrons, and thus the aforementioned caste of electron-electron repulsion and shielding) with differing numbers of protons (and thus different nuclear attraction), giving the relative ionic sizes of each atom or ion.
The Periodic Trend
Due to each atom's unique power to lose or proceeds an electron, periodic trends in ionic radii are not as ubiquitous every bit trends in atomic radii across the periodic table. Therefore, trends must be isolated to specific groups and considered for either cations or anions.
Consider the southward- and d-cake elements. All metals can lose electrons and form cations. The alkali and alkali earth metals (groups ane and 2) grade cations which increase in size down each grouping; atomic radii conduct the same manner. Beginning in the d-block of the periodic table, the ionic radii of the cations do not significantly change across a period. Nonetheless, the ionic radii do slightly decrease until group 12, after which the tendency continues (Shannon 1976). It is important to note that metals, not including groups 1 and 2, can have different ionic states, or oxidation states, (east.g. Fe2 + or Fe3 + for iron) so caution must exist employed when generalizing about trends in ionic radii across the periodic table.
All non-metals (except for the noble gases which do not form ions) form anions which become larger downward a group. For not-metals, a subtle trend of decreasing ionic radii is found beyond a pegroup theoryriod (Shannon 1976). Anions are almost always larger than cations, although there are some exceptions (i.e. fluorides of some alkali metals).
Measurement and Factors Affecting Ionic Radii
The ionic radius of an cantlet is measured by calculating its spatial proportions in an ionic bond with another ion inside a crystal lattice. However, it is to consistently and accurately determine the proportions of the ionic bonds. After comparing many compounds, pharmacist Linus Pauling assign a radius of 140 pm to Otwo- and utilise this as a reference bespeak to determine the sizes of other Ionic Radii (Jensen 2010).
Ionic radius is not a permanent trait of an ion, but changes depending on coordination number, spin state, and other variables (Shannon 1976). For a given ion, the ionic radius increases with increasing coordination number and is larger in a high-spin state than in a low-spin land.
According to group theory, the idea of ionic radii as a measurement of spherical shapes simply applies to ions that course highly-symmetric crystal lattices like Na+ and Cl -. The point group symmetry of a lattice determines whether or not the ionic radii in that lattice can be accurately measured (Johnson 1973). For case, lattices with Oh and Td symmetries are considered to have high symmetry; thus the electron densities of the component ions occupy relatively-spherical regions and ionic radii can exist measured fairly accurately. However, for less symmetrical and more than polar lattices such equally those with Cdue north, Cnh, and Cnv symmetries, significant changes in the electron density can occur, causing deviations from spherical shape; these deviations brand ionic radii more difficult to measure.
References
- Housecroft, Catherine E. & Alan G. Sharpe. Inorganic Chemistry. threerd ed. England: Pearson Pedagogy Limited, 2008.
- Shannon R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica 1976;32(5): 751-767.
- Jensen B., William. The Origin of the Ionic-Radius Ratio Rules. Journal of Chemical Education 2010;86(6):587-8.
- Oliver, Johnson. Ionic Radii for Spherical Potential Ions. Inorganic Chemistry 1973;12(4):780-85.
- Birkholz, Mario. Crystal-field induced dipoles in heteropolar crystals II: Physical significance. Z. Phys. 1995;96:333-twoscore.
Problems
- Why are cations smaller and anions larger than their respective atoms?
- Cations are smaller than their respective atoms because of increased electron-electron repulsion
- Anions are larger than their respective atoms because of increased effective nuclear charge
- Cations are smaller than their respective atoms because of decreased electron-electron repulsion
- Anions are larger than their corresponding atoms considering of decreased effective nuclear charge
- Which of the following are isoelectronic: \(F^-\), \(Cl^-\), \(Ca^{2+}\), \(Ar\)
- List the ions from smallest to largest: \(Se^{2-}\) ,\(Zr^{4+}\), \(Na^+\),\(Mg\), \(Rb^+\), \(Br^-\), \(K^+\)
- How are ionic radii measured?
- What are some of the problems with generalizing ionic trends?
Solution
- C & D: Cations are formed when an electron is lost. When this occurs at that place are less electron-electron repulsions and there is a greater net nuclear allure per electron. And then, the newly formed ion becomes a more condensed version of its neutral atom. Anions are formed when an electron is gained. When this occurs there are more than electron-electron repulsions and at that place is a lower net nuclear allure per electron. This will cause the electrons push each other abroad and spread out, causing the atom to become larger.
- Cl-, Ca2 +, Ar all have 18 electrons; therefore, they are isoelectronic (F- has 10 electrons). An isoelectronic series is useful in understanding the effects of gained or loss electrons on atom size.
- Zriv +<Grand+<Rb+<Mg<Br-<Seii-: Ionic radii shorten with increasing positive accuse and lengthen with increasing negative charge, and thus, anions are almost e'er larger than cations.
- Ionic radii are measured by proportioning ionic bond lengths betwixt two ions inside a crystal lattice. Later studying many compounds, Linus Pauling found that the approximate ionic radii of O2- was 140pm. With this reference signal, Pauling was able to summate the ionic radii of other ions.
- Ionic radii are not fixed properties of ions. For the same ion, the radii tin can differ in different crystal lattices due sure variables such as coordination number and electron spin. Group Theory suggests that only ions in loftier-symmetric non-polar crystal lattices can accurately be measured for their radii.
Contributors and Attributions
- Michael H. Nguyen. University of California, Davis
Source: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Periodic_Trends_of_Elemental_Properties/Periodic_Trends_in_Ionic_Radii
0 Response to "what happens to the radius as an atom changes to an anion ion"
Post a Comment